Advanced functional imaging in oncology is essential in decision making for cancer care. As our understanding of the biology and pathogenesis of cancer improves, the impact of functional imaging will become increasingly important. Research into the standardisation and automation of PET/CT criteria in determining treatment response in cancer are vital.
Cancer is a significant cause of mortality worldwide. A critical component in the clinical management of cancer is advanced imaging modalities including PET, CT and MR. Imaging in oncology is essential in the diagnosis, staging, prognosis, treatment planning, treatment delivery, treatment response assessment, and ultimately decision making for cancer care. As the oncologists' understanding of the complex biology of tumour response and functional status has evolved, so have the abilities to integrate these facets with imaging. Along with this evolution, there has been a progressive recognition that quantitative methods can be applied to the resultant images and that quality control and robust computerised approaches to measurement can improve the accuracy of interpretation. However, there is simultaneously a growing impetus for cost-effective, cost-conscious care that provokes the need for standardisation of imaging techniques and automated measurement tools to reproducibly measure efficacy and response in clinical trials and cancer care more generally. These changes in oncology healthcare practice have the potential to improve outcomes and reduce global resource utilisation.
The article reviews current standards for image-based treatment response assessment in cancer based on anatomic criteria alone, generally with CT or MR scans using the RECIST criteria. The discussion then centres on the role functional imaging techniques might play in response assessment and specifically the role that FDG-PET/CT can have on evaluation of treatment response in cancer. Identification of the current and future applications of the PET/CT imaging technique is provided. Some practical clinical examples of PET/CT utilisation for evaluation of treatment response are depicted and described. In addition, developing tools for the standardisation and automation of quantitative metrics using PET/CT with global application are discussed. Hence, the current impact and future implications of PET/CT imaging in the treatment assessment of cancer are discussed and addressed.
Current standards of image-based treatment response
The most common metric to describe tumour response is defined by the RECIST Criteria. This method uses cross sectional anatomic imaging, most often CT, to define the single longest dimension of the tumour. This is determined by the radiologist who is generally using a measurement tool and is subjectively defined by that physician. More recent analyses have employed the sum of the two longest dimensions in any two orthogonal planes. Based on slice differences on subsequent follow-up scans as well as the imperfect measurement paradigm, RECIST categories for response are relatively imprecise. These categories include Complete Response (CR): disappearance of all tumour foci for at least four weeks; Partial Response (PR): a decline of at least 30 per cent in the tumour diameter for at least four weeks; Stable Disease (SD): neither partial response nor progressive disease; and Progressive Disease (PD): at least a 20 per cent increase in the sum of tumour diameter from the initial tumour size. These criteria are then generally applied to determine whether or not therapy is to be continued or not: success versus failure of therapy. Assessment of five to ten lesions is a standard protocol used for metastatic disease.
Although the RECIST criteria are well established and practically acceptable and achievable, there are limitations in its ability to accurately and reproducibly define an image-based treatment response. The potential for misclassifications and variance in response is more apparent when a different reader completes the initial and subsequent follow up studies, although repeated measurements by the same reader on different days also often reveals discrepancies. In addition, the RECIST criteria do not completely measure all the biologic and metabolic changes manifest within the cancer that may further define and determine a more accurate imaged-based treatment response assessment. It is well recognised that inflammatory responses may increase the size of lesions which can then be followed by lesion involution. The need for evaluating treatment response beyond anatomic measurement is evident and has led to research and development of quantitative functional-based imaging to assess metabolic and biologic parameters in cancer for more accurate response assessment.
Quantitative Imaging using PET/CT for response assessment
A prime example of quantitative imaging that combines the functional metabolic sensitivity of PET and the temporal and spatial resolution of CT is PET/CT imaging. Applications of PET/CT imaging have become a standard response assessment tool for many cancer sites, and further more, in initial diagnosis and staging. A key advantage of PET/CT imaging is its ability to evaluate treatment response through quantitative assessment, measurement, and understanding of the metabolic activity in a tumour. In addition, changes in the tumour volume that may or may not correlate with outcome can be viewed with knowledge of the associated metabolic changes. Its clinical impact and relevance reside in determining how the measurement and change in metabolic activity in PET/CT imaging after treatment correlates to overall clinical response, prognosis, survival, toxicity, and morbidity in the individual cancer patient. Significant data analysing the role of FDG PET in head and neck cancer response assessment are well published.
Most imaging-based response with PET/CT is based on the tracer 18-FDG radioactive isotope, which is an analogue of glucose and the most commonly used PET tracer. This analogue is transported across the cell membrane through facilitated transport proteins such as glut 1 and glut 3 down a concentration gradient. These transport proteins are over-expressed in tumour cell membranes compared to normal tissues. The phosphorylated form of FDG cannot be further metabolised and consequently accumulates in cells after measured glycolytic activity and energy expenditure. FDG-PET uptake in tumours correlates with high rates of glycolysis, far greater than in normal tissues. These high rates of glycolysis and consequent uptake are likely indicative of the tumour's biology and behaviour.
The most common measurement of tumour metabolic activity used by PET is the Standardised Uptake Value (SUV). It is defined by tumour activity per dose injected per body mass. SUV is proportional to the metabolic rate of glucose in normal tissue when a normal serum glucose concentration is present. A metabolic response within the tumour is most commonly defined by the percentage change of post-therapy SUV from the pre-therapy SUV. The relative percentage change and the maximum SUV value and/or cutoff may serve as predictive and prognostic markers in cancer. However, an optimally accurate, standard, and reproducible means to capture the relationship between tumour metabolic activity and treatment response through PET/CT imaging is yet to be fully described and determined.
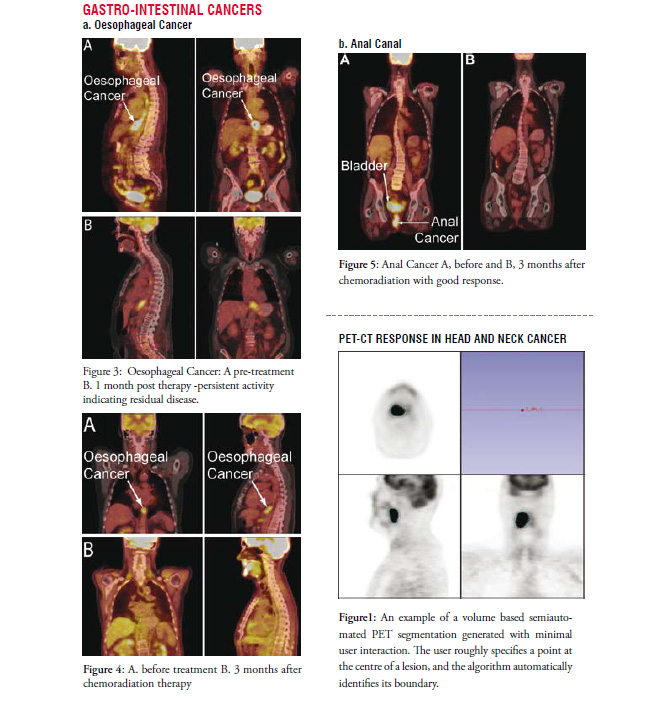
Whether a maximum value, a mean value or a metabolic tumour volume is most important is not entirely clear. In addition a full analysis of potential imaging features within the vast amount of information contained in a volumetric PET image with a dynamic character of FDG uptake is poorly studied. Future development of robust tools that include detailed analysis of a spectrum of imaging features when combined with bio-informatics and outcome data is highly likely to lead to improvement in the predictive value of PET/CT. An example of a semi-automated tumour contour on a PET/CT image derived from a novel algorithm is shown in Figure 1. Such methods have the promise of more consistently identifying metrics within a scan that will enable more consistent comparisons for tumour response. Furthermore, the algorithms make robust analysis more feasible.
Standardisation and automation of PET/CT imaging in cancer treatment response
While functional imaging, as illustrated by FDG PET/CT, better captures the complex nature and biology of cancer and improves our understanding of treatment response based on associated change in tumourmetabolic activity, there is no standardised criteria and means to quantify the exact treatment response and its clinical implications in a reproducible, automated, and cost-effective manner.
Appropriate patient preparation, image acquisition, data transfer, and post-processing data analysis are necessary components in order to develop a robust approach in the measurement and assessment of treatment response with PET/CT imaging. There is also a need for automated, objective, quantitative metrics to differentiate true tumour cell killing from other physiologic changes such as inflammation, infection, cell injury, fibrosis, etc. As described above, a potential quantitative measurement of early treatment induced changes and post-treatment response is the SUV. The determination of SUV is dependent on identical patient preparation and adequate scan quality that is similar between baseline and follow up studies. Furthermore, imaging should be capable of producing exactly the same quantitative measurement for a given uptake on one day versus another and on one scanner versus another. A standardised phantom for absolute calibration of PET/CT scanners has been lacking. Working together through the Quantitative Imaging Network funded by the National Cancer Institute there are now new ways being developed to achieve this endeavour such basic tools for standardisation as well as for automated analysis will be critical in the future application of imaging to clinical trials.
While imaging is expensive today, the cost of delayed determination of treatment efficacy or lack thereof is substantial both in human terms to the individual patient as well as in the economic cost of continued therapy that may be ineffective. The increased utilisation of functional imaging and in particular PET/CT for cancer is driving both the development of improved tools for application as well as the study of optimal protocols for imaging the response in terms of the timing of scans relative to therapy and the specific agents that are being applied. The combination of possibilities is large and the potential for improvement is great with the promise of a non-invasive way to both predict the best therapy for patients as well as early determination of effectiveness. This will lead to personalised therapies based on these functional image based responses.
Conclusions
Functional imaging is a critical component in the delivery and management of optimal oncologic healthcare of the individual cancer patient. As the oncologists' understanding of the complex biology and pathogenesis of cancer improves, the impact of functional imaging and its many applications will increase and become even more paramount. The future of PET/CT imaging is indeed bright especially in the improved evaluation of treatment response in current oncologic practice. Its potential to become a critical component and biomarker in future clinical trials and the delivery of minimally invasive molecular targeted therapies with the least toxicity and morbidity can both preserve quality of life and decrease cost-expenditures. Increased awareness and heightened research in the standardisation and automation of PET/CT criteria in determining treatment response in cancer are essential and will drive the future of oncology care for patients worldwide.